|
The
labeled flask
|
||||
|
A
|
B
|
C
|
D
|
|
| Volume of acid used(ml) |
80
|
35
|
35
|
35
|
| Starting temperature of the solutions (oC ) |
18
|
70
|
18
|
5
|
The volume of carbon dioxide produced was measured every minute and the results shown in the graph below.
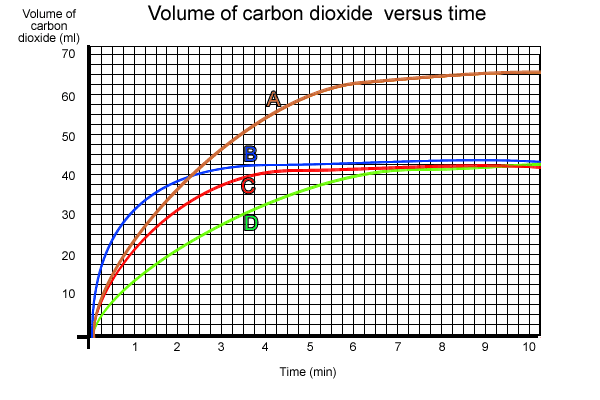
1) Which flask produced the
most carbon dioxide at 1 minute?
2) Explain why flasks "B", "C" and "D" produced
less carbon dioxide than flask "A".
3) Irene was heard to comment "The experiment shows that the amount
of carbon dioxide produced after ten minutes depends on the temperature."
Is this correct?
4) What is the effect of temperature on the reaction?
Solution